Fuji Diosynth - Virtual Tour

Welcome to FUJIFILM Diosynth Biotechnologies, located in Research Triangle Park, North Carolina, we operate a 24 hours 7 days per week cGMP facility as a non-sterile, bioburden-controlled, multi-product facility capable of fermentation, cell culture, recovery, purification, and bulk filling. Our facility operates in compliance with applicable US, EU, and ROW cGMP regulations. In addition to manufacturing the facility includes: QC laboratories, warehousing, waste management, and plant utility operations.

Lobby
FUJIFILM Diosynth Biotechnologies is a Global Contract Development and Manufacturing Organization (CDMO) specializing in Biopharmaceutical Process Development and production of Bulk Drug Substance.
Our 62,000 square feet cGMP production facility is located on our 69 acres Research Triangle Park, North Carolina campus.

About our GMP Purification Suites
We have 5 purification suite areas available to support upstream production and these suites can operate at ambient and chilled temperatures.
- All purification suites operated at Grade C
- Bulk filling is performed in a hood under terminal HEPA filters tested to Grade A requirements
- Scaled-down models to support viral validation studies
- Extensive experience with inclusion bodies and refolding
- Expertise in protein PEGylation and other modifications
GE AKTA Ready
- This is a Single-Use chromatography system.
- Designed for use with traditional or pre-packed disposable columns
- Faster equipment set up times and reduces cleaning requirements

Purification Suite 2
Our Purification capabilities include both traditional and single-use trains.
We have ÄKTA process Chromatography Skids for process scale-up and for large-scale manufacturing. Attributes include:
- Dual pump system to enable gradient elutions
- Configured with UNICORN control software that scales lineraly from process development into manufacturing
Cold Room Capabilities
We have:- Dual cold room capability for 2-8°C processing
- Our cold rooms typically house a large chromatography skid

Cell Culture 1
Our cell culture manufacturing capabilities include both single use and traditional stainless steel capabilities. These include 1,000L and 2,000L Single Use Suites with associated smaller seed bioreactors consisting of rocker systems and 50L and 500L single use bioreactors. The 2,000L stainless steel production bioreactor includes a 145L and 650L train. Our cell culture suites are serviced by dedicated air handlers and dedicated personnel flow.
Here is a look at our Traditional 2,000L stainless steel GMP Cell Culture
- Two stage seed reactor train (145L and 650); seed reactors can be operated as production reactors
- Powder media makeup and liquid media filtration
- Automated SIP and CIP
- Production bioreactor with marine impeller and macro-sparge gassing platform
- Hundreds of commercial batches produced
- Operated at Grade D with independent HVAC system

Cell Culture 2
We have:
- Two stage seed reactor train (50L and 500L) both of which can be operated as production reactors
- Highly scalable reactor systems with excellent mixing and gas transfer
- Systems leverage our commercial stainless steel bioreactor gassing strategies
- Systems support high density CHO and Insect cell cultures with robust process control
- Powder media makeup and liquid media filtration
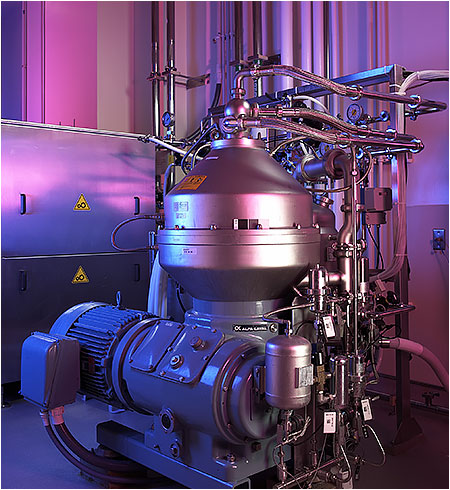
Cell Culture Harvest
- Capabilities for single-use depth filtration and disc-stack centrifugation
- 1,500L product collection vessel

Microbial Inoculum Room
We have:
- Two (2) Microbial Inoculum suites with media make-up and autoclaving capabilities
- Biosafety cabinets for vial thaw and aseptic transfers
- Two (2) state-of-the-art 30L seed fermentors with full automation control and feed control systems
- Equipped with full trending and alarm functions to ensure each batch meets client specifications
- Operated at Grade D with independent HVAC system
Entrance to our dual 2,000L GMP Fermentation Suites
- Dual 2,000L fermenters with a single 200L seed fermenter
- Fully automated SIP and CIP systems
- Fully automated control schemes with capability for multiple feed strategies
- Fermenters with excellent oxygen transfer and cooling capability that enables high density and highly active cultures
- Explosion proof MeOH feed systems
- Hundred of commercial batches produced

Fermentation - Small Scale
We have extensive expertise with microbial-based processes for manufacture. This experience includes clinical and commercial production from E.coli and P.pastoris hosts as well as clinical manufacture using a wide range of other microbial systems.
We have small and intermediate scale cGMP microbial capabilities. Our intermediate scale cGMP fermentation capabilities include two (2) separate production suites including a 1 X 2,000L train and 2 X 2,000L train.
Our small scale cGMP fermentation capabilities include:
- 140L stainless steel fermenter
- Dedicated Inoculum room with Biosafety cabinets for vial thaw and aseptic transfers
- Operated at a Grade D environment and utilizes 100% fresh air delivered by HVAC